

Professor William Epling, who chairs the Department of Chemical Engineering at the University of Virginia School of Engineering and Applied Science, has one very good reason you should care about a process that scientists refer to as “catalysis.”
“When it comes to climate change, chemical engineers are going to be key in solving it one way or the other,” he said. “If you care about the environment, you should care about catalysis.”
So, what does the word mean, how can it help the environment, and what is the future of the field in terms of employment and UVA’s own efforts?
Epling explains catalysis in the following listicle, including how UVA is contributing to the fight to help our planet sustain life:
- A catalyst isn’t a domino. It’s more like a dial.
In popular culture, we often refer to a catalyst as something that sets off a big chain of events. In chemical engineering, however, that’s not a catalyst.

A catalyst isn’t a domino. It’s more like a dial.
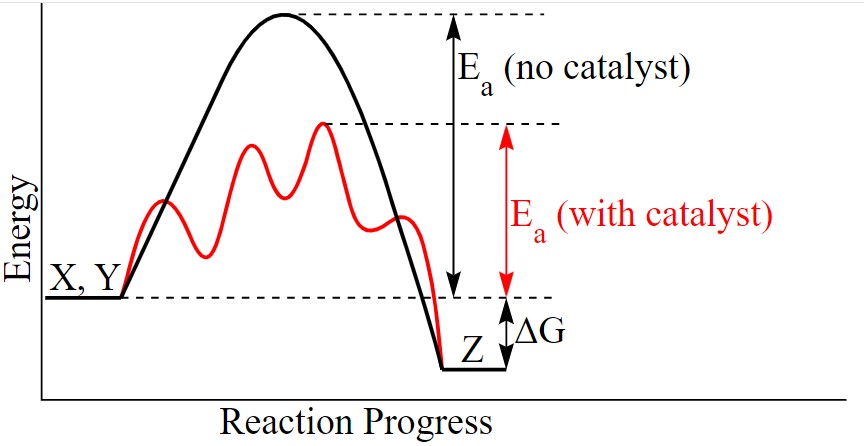
“Catalyst” is defined as a substance that speeds up a reaction without being used up as part of the reaction. Thus, “catalysis” is the process of using a catalyst to achieve a desired end. Chemical engineers in industry catalyze reactions to produce industrial products as efficiently as possible, saving time, energy and money.
More specifically, a catalyst lowers the activation energy needed to break apart molecular bonds and recombine them into new forms. The process of catalysis can also lower the reaction temperature or the pressure needed to obtain certain economic product yields, which often means benefits to society.
2. Catalysis is an integral part of all of our lives.
Catalysis plays a big role in how the world and its industries utilize chemistry to societal advantage, often creating affordable products for everyday needs and, by association, jobs.

When people think of industrial chemistry and chemical engineering, petroleum-based products often come to mind, such as certain types of plastic, or maybe commercial chemicals such as ammonia. But a wide range of household products, much of our clothing, even our groceries — such as bread, beer and wine made with yeast — are among the countless consumer products that rely on catalysis.
In fact, catalysis is an integral part of all of our lives, even if we don’t purchase a product. Our bodies rely on the process. Catalysts called enzymes digest the food we eat so that the nutrients can be quickly absorbed by our cells, and they help us rebound sooner after injury.
But many chemical reactions not only create unwanted pollutants, but speed up climate change. Short of banning the creation of a chemical or its processing, which is always an option via regulation, the solution is to try to manufacture it more efficiently using catalysis.

3. Engineers are always looking for ways to make products more efficiently, which often means greener.
Catalysis extends to all things synthetic. Engineers are always looking for ways to make these products more efficiently, which often means greener. And chemical engineers in this field are looking to find the smallest amount of catalyst that can provide the greatest return on investment. Sometimes that’s easier said than done.
While catalysts don’t get used up in reactions, their efficiency can be lessened over time by the products or byproducts of a reaction, or from other things coming in with the feed.
4. The field of chemical engineering is growing, in part thanks to catalysis.
Overall, the demand for chemical engineers is expected to grow by 8% over the next decade, according to recent data from the Bureau of Labor Statistics. The reason is the combination of global industrial growth and continued concerns about mitigating environmental impact.
Yes, the field of chemical engineering is growing, in part thanks to catalysis. An undergraduate degree in chemical engineering is currently considered sufficient to gain entry into the field.
5. UVA is helping the environment by investing in catalysis solutions.
Three years ago, the Office of the Vice President for Research announced that UVA would be applying $60 million toward solutions in the fight against climate change, primarily through translatable technologies that help the environment by reducing carbon dioxide in the atmosphere. Since then, UVA chemical engineers have partnered on projects internally and with their chemistry peers in the School of Arts & Sciences, either with direct funding from UVA or facilitated by the broader research investments of lab equipment and an increased graduate and postdoctoral research workforce.
Whether by making products more efficiently, or cleaning emissions up on the back end, UVA is helping the environment by investing in catalysis solutions.