Research
We develop new or improved catalytic materials by studying how the structure of a catalyst affects its performance in chemical reactions.

Reactions of Dioxygen on Isolated Transition Metal Ions in Nitrogen-Doped Carbon
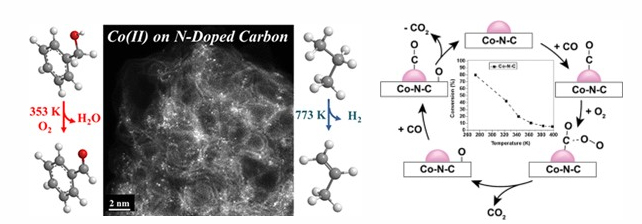
Recent work from our group has focused on understanding how an isolated metal ion in nitrogen-doped carbon (M-N-C) catalyzes reactions involving O2. These M-N-C materials are known to be highly active for the electrochemical oxygen reduction reaction and oxidative dehydrogenation reaction with many research papers appearing in the last decade. Nevertheless, the nature of the active site and mechanism of O2 activation remain elusive. Our group has investigated thermal oxidation reactions over M-N-C catalysts in an attempt to count sites and elucidate the mechanism for O2 activation.
Our synthesized materials were characterized with a variety of methods including X-ray absorption spectroscopy, aberration corrected high-angle annular dark-field scanning transmission electron spectroscopy, and X-ray photoelectron spectroscopy. In addition, acid titration experiments were performed to try to analyze the number of active sites, which indicated a rapid turnover frequency for benzyl alcohol oxidative dehydrogenation, similar to that over a Pt metal catalyst. To estimate the turnover frequency of low temperature CO oxidation over Co-N-C without a priori knowledge of the active site density, steady-state isotopic transient kinetic analysis (SSITKA) was performed. The transient analysis revealed a very rapid turnover frequency and low surface coverage of adsorbed intermediates leading to product CO2.
ACS Catal. 8 (2018) 3875-3882 ACS Catal. 12 (2022) 15529-15540
Reaction kinetics measured during low temperature CO oxidation over Co-N-C were consistent with both the CO and O2 being bound weakly to the surface during the reaction. Results from experiments coupled with quantum chemical density functional theory calculations (in collaboration with Dr. Christopher Paolucci at UVA) supported a reaction path with both CO and O2 co-adsorbed on the isolated Co ion and the carbon support. On-going work is exploring the generality of the mechanism of CO oxidation on other M-N-C materials.
Recent Publications
C. A. Whitcomb, A. Sviripa, M. I. Schapowal, K. Mamedov, R. R. Unocic, C. Paoluci, R. J. Davis, “Mechanistic Insights on the Low-Temperature Oxidation of CO Catalyzed by Isolated Co Ions in N-Doped Carbon” ACS Catal. 12 (2022) 15529-15540
J. Xie, J. D. Kammert, N. Kaylor, J. W. Zheng, E. Choi, H. N. Pham, X. Sang, E. Stavitski, K. Attenkofer, R. R. Unocic, A. K. Datye, R. J. Davis “Atomically Dispersed Co and Cu on N-Doped Carbon for Reactions Involving C-H Activation” ACS Catal. 8 (2018) 3875-3884.
J. Xie, K. Yin, A. Serov, K. Artyushkova, H. N. Pham, X. Sang, R. R. Unocic, P. Atanassov, A. K. Datye, R. J. Davis, “Selective Aerobic Oxidation of Alcohols Over Atomically-Dispersed Non-Precious Metal Catalysts” ChemSusChem 10 (2016) 359-362.
CO2 Methanation for Biogas-to-Renewable Natural Gas
Biogas from various sources including wastewater treatment plants, landfills, and anaerobic digestion facilities can serve as a resource to produce renewable natural gas (RNG). Conventional biogas upgrading technologies involve removing CO2 from CH4 streams, which is operationally intensive and substantially lowers the overall carbon yield of the process. To overcome these challenges, we are investigating a low-temperature thermocatalytic process to convert CO2 in biogas to CH4, producing pipeline-quality RNG. The core component in this process is a highly active, durable, and selective catalytic CO2 methanation reaction unit operating at relatively low temperatures and low pressures to be economically viable. Research focuses on synthesizing highly promoted supported Ni catalysts and evaluating their catalytic performance for the conversion of model biogas. The life cycle and techno-economic analyses associated with the production of RNG are also being explored. This project is performed in collaboration with Professor Sen Zhang in the Chemistry Department and Professor Lisa Colosi Peterson in the Civil and Environmental Engineering Department at UVA and researchers at the National Renewable Energy Laboratory.
Mutifunctional Catalysts for the Conversion of Oxygenates
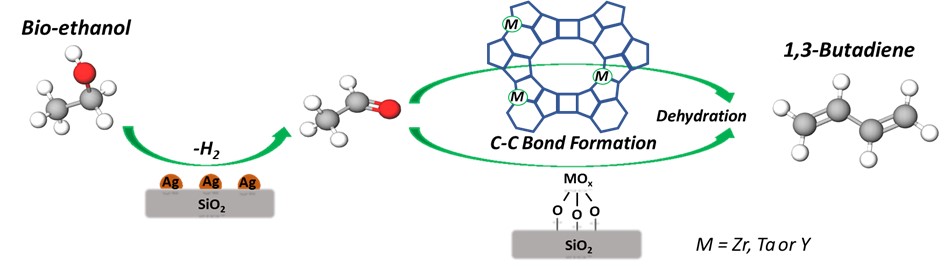
Recent work from our group has focused on the use of multifunctional catalysts, composed of supported metal nanoparticles and supported early transition metal oxides, that demonstrate the viability of upgrading light alcohols to higher value chemicals. For example, the Lebedev reaction is of considerable interest as it converts ethanol to 1,3-butadiene, a chemical used in the production of synthetic rubber and plastics. Conversion of ethanol to butadiene requires a complex cascade of reactions that includes alcohol dehydrogenation, C-C bond formation, hydrogen transfer, and dehydration. Our recent work explored the multifunctional catalyst composed of Ag nanoparticles combined with silica-supported ZrO2. Ongoing work explores the importance of silica-supported and zeolite-supported oxides of Zr, Y, and Ta in the Lebedev reaction mechanism. Characterization of the materials involves a wide variety of methods, including X-ray absorption spectroscopy, UV-vis spectroscopy, X-ray photoelectron spectroscopy, IR spectroscopy, and X-ray diffraction. In addition, catalytic probe reactions that are sensitive to the acid-base nature of the supported oxides are used in an effort to relate structure and composition to reactivity.
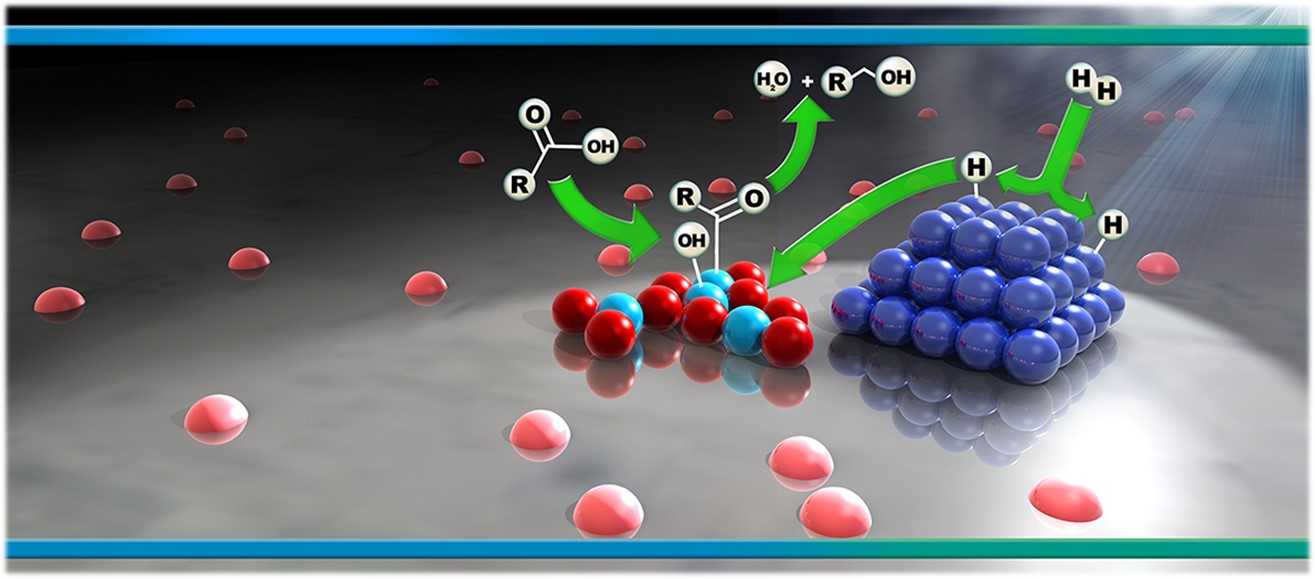
Multifunctional catalysts are used in the reduction of carboxylic acids with H2 to form aldehydes and alcohols, which are important in the production of oils, lubricants, and detergents. Here we explore hydrodeoxygenation of carboxylic acids over metal-promoted reducible metal oxide clusters such as Pd-promoted ReOx or WOx supported on silica. During catalytic reaction, the hydrogen serves as a reductant for the acid but also forms the active catalyst via hydrogen spillover from Pd to ReOx or WOx. This cooperative nature of the metal and metal oxide within these multifunctional catalysts is probed through steady-state and transient kinetics of probe reactions as well as spectroscopic analyses. This experimental work is performed in parallel with ab initio quantum chemical studies carried out in the lab of Professor Christopher Paolucci.
Recent Publications
K. Mamedov and R.J. Davis, “Cascade Reaction of Ethanol to Butadiene over Ag-Promoted, Silica- or Zeolite-Supported Ta, Y, Pr, or La Oxide Catalysts” ACS Catal. (2023) 13 (5), 3333-3344
N. Miyake, G. Brezicki, and R.J. Davis, “Cascade Reaction of Ethanol to Butadiene over Multifunctional Silica-Supported Ag and ZrO2 Catalysts” ACS Sus. Chem. Eng. (2022) 10 (2), 1020-1035
J.T. Prillaman, N. Miyake, R.J. Davis, “Calcium Phosphate Catalysts for Ethanol Coupling to Butanol and Butadiene” Catal Lett. (2021) 151, 648–657
J.D. Kammert, A. Chemburkar, N. Miyake, M. Neurock, and R.J. Davis, “Reaction Kinetics and Mechanism for the Catalytic Reduction of Propionic Acid over Supported ReOx Promoted by Pd” ACS Catal. (2021) 11 (3), 1435-1455
J.D. Kammert, G. Brezicki, N. Miyake, E. Stavitski, R.J. Davis, “Reduction of Propanoic Acid over Pd-Promoted Supported WOx Catalysts” ChemCatChem (2020) 12, 314-325
J.D. Kammert, J. Xie, I.J. Godfrey, R.R. Unocic, E. Stavitski, K. Attenkofer, G. Sankar, and R.J. Davis, “Reduction of Propionic Acid over a Pd-Promoted ReOx/SiO2 Catalyst Probed by X-ray Absorption Spectroscopy and Transient Kinetic Analysis” ACS Sus. Chem. Eng. (2018) 6 (9), 12353-12366
Z.D. Young and R.J. Davis, “Hydrogen Transfer Reactions Relevant to Guerbet Coupling of Alcohols over Hydroxyapatite and Magnesium Oxide Catalysts,” Catal. Sci. Technol. (2018) 8, 1722-1729
S. Hanspal, Z.D. Young, J.T. Prillaman, R.J. Davis, “Influence of Surface Acid and Base Sites on the Guerbet Coupling of Ethanol to Butanol over Metal Phosphate Catalysts,” J. Catal. (2017) 352, 182-190
Center for Bioenergy Innovation
We have recently joined the Center for Bioenergy Innovation (CBI) working on catalytic upgrading of bioethanol to sustainable aviation fuels. You can read more on the CBI website LINKED HERE.