Life's Cosmic Handshake: When Chiral Surfaces Meet Chiral Organic Molecules
(NASA - FINESST | 2022-2024)
The almost exclusive usage by organisms of left-handed amino acids and right-handed sugars – a feature called homochirality – continues to elude explanation and remains an enigma in the story of the origin of life. However, the detection of an enantiomeric excess of left-handed (L-ee) amino acids and right-handed sugars in carbonaceous meteorites strongly points to a cosmic origin. This is perplexing since non-biological reactions usually lead to a racemic mixture. Mechanisms for asymmetry include: chance, asymmetric absorption of circularly polarized light (CPL), and the weak nuclear force. However, all of these explanations come up short in explaining the measured magnitude of enantiomeric excess in meteorites. Analyses of meteorite samples show that many amino acid enantiomers are in excess compared with the expected racemic distribution. The excess is almost always found in favor of left-handed amino acids, the type used by life.
 Laboratory experiments utilizing CPL have been able to produce an enantiomeric excess of just a few percent, unlike the double-digit excesses for some amino acids in meteoritic material. Subsequent aqueous alteration, correlated to L-ee in carbonaceous meteorites, has been linked to the amplification of excess levels. However, CPL should affect all species of amino acids, yet not every amino acid in meteorites show an excess. Indeed, polyols—sugar alcohols—in the same meteorite samples show the opposite handedness and, in many cases, 100% preference for the right-handed enantiomer. Lastly, the Paris meteorite, potentially the best representative of the presolar nebula, has racemic mixtures of amino acids. Clearly, CPL is not the only factor at play.
Laboratory experiments utilizing CPL have been able to produce an enantiomeric excess of just a few percent, unlike the double-digit excesses for some amino acids in meteoritic material. Subsequent aqueous alteration, correlated to L-ee in carbonaceous meteorites, has been linked to the amplification of excess levels. However, CPL should affect all species of amino acids, yet not every amino acid in meteorites show an excess. Indeed, polyols—sugar alcohols—in the same meteorite samples show the opposite handedness and, in many cases, 100% preference for the right-handed enantiomer. Lastly, the Paris meteorite, potentially the best representative of the presolar nebula, has racemic mixtures of amino acids. Clearly, CPL is not the only factor at play.
Amino acids in the early solar nebula are expected to derive from photolysis/radiolysis of simple condensed gases, with subsequent sublimation and eventual redeposition onto proto-planetary grains. Most laboratory experiments involving amino acid redeposition ignore the surface effects of the substrate. However, chiral mineral surfaces might be fundamental in enhancing one enantiomer over another via asymmetric desorption in the protoplanetary disk. The desorbed enantiomer could then be destroyed in a myriad of processes, leaving the surface with a bias in the observed enantiomeric excess. Furthermore, not all chiral organic molecules would be affected the same way, thus providing an explanation for why some amino acids are racemic and others show an enantiomeric excess.
We propose a single-year combined computational and experimental approach to test whether astrophysically relevant chiral surfaces can enhance the appropriate enantiomers of each class of biological molecules. First, we suggest detecting the functional groups involved in surface binding of alanine, tyrosine, and glutamic acid enantiomers on (101) quartz in an astrophysically relevant laboratory environment. Quartz is used a model chiral mineral and the amino acids were chosen so that a diverse set of functional groups are represented. The experimental setup consists of an UHV chamber (~10-10 Torr) with an X-ray photoelectron spectrometer (XPS), mass-spectrometer, hot-cold stage, and adjacent sample introduction chambers. Preliminary experiments have been run with tyrosine and glutamic acid aqueously adsorbed onto quartz. XPS data is used to determine which functional groups are utilized in surface bonding. The preliminary experiments show subtle differences between each enantiomer of the amino acids tested. Angle-resolved XPS can be used to determine the orientation of the amino acid on the surface.
Density functional theory (DFT) calculations using the Vienna ab initio Simulation Package (VASP) will be used to interpret surface kinetics. The VASP package has been previously used to predict enantiomeric differences in surface binding energies for chiral molecules on chiral surfaces. Energy calculations will be conducted for a suite of chiral organic molecules; (101) quartz; and (110) enstatite – an astrophysically relevant mineral with chiral faces. Various orientations of those amino acids with respect to the proposed surfaces will be studied to determine the desorption energies. We hypothesize that the difference in binding energies between organic molecule enantiomers on chiral mineral surfaces is large enough that sublimation from such surfaces may play an important role in enhancing the enantiomeric excess of the molecules during planetary formation. If this hypothesis is correct then one important test that of it is to show that the preference for amino acid enantiomers and polyol enantiomers must be in opposite handedness.
PI: Catherine Dukes (Research Scientist)
FI: Kamil Stelmach (PhD Graduate Student)
UVa Contributors: Kamil Stelmach (Chemistry PhD Student); Rob Garrod (Project Co-I/Chemistry Professor).
Proceedings and Publications:
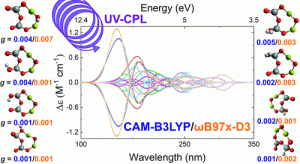
K.B. Stelmach, C.A. Dukes, and R.T. Garrod, " A Search for Chirality in Hydrogenated Magnesium Nanosilicates: A DFT and TD-DFT Investigation," Journal of Physical Chemistry A, 128, 18, 3475–3494, (2024) https://doi.org/10.1021/acs.jpca.3c06521
K. B. Stelmach, C.A. Dukes, and R.T. Garrod, “Chiral Enstatite Nanostructures and their Potential Relevance to Homochirality: DFT and TD-DFT Calculations,” AbSciCon 2024, 5-10 May, Providence, RI, ID#XXXXX (2024)
K.B. Stelmach, C.A. Dukes, R.T. Garrod, "Life’s Cosmic Handshake: Symmetry Breaking in Enstatite Nanosilicates," Fall AGU23 ID# 1415760 (2023)
K.B. Stelmach, C.A. Dukes, & R.T. Garrod, "LIFE’S COSMIC HANDSHAKE: DFT AND TD-DFT PREDICTIONS FOR THE PROPERTIES OF ENSTATITE (MgSiO3) MONOMERS AND DIMERS," 76th International Symposium on Molecular Spectroscopy, June 19-23, Urbana, Il , #P7017 (2023)
K.B. Stelmach, C.A. Dukes, and R.T. Garrod, Adsorption of Tyrosine and Glutamic Acid to the Chiral (101) Face of Quartz: An XPS Study, 52nd LPSC Abst# 2685 (2021)