MSE Undergraduate Courses
Explore below to learn more about our MSE undergraduate courses...
Materials Science?
- Could we, and should we, make a bridge out of glass?
- Why can we write with graphite, but not with diamond? They’re both just carbon…
- Airplanes are made of aluminum. Why aren’t cars?
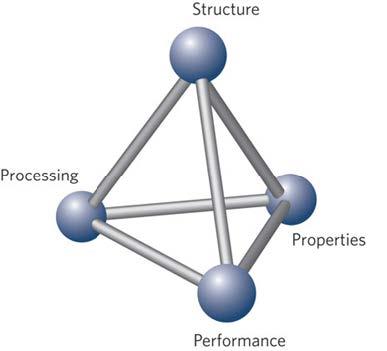
Materials are used to construct every technology used by our society, spanning the seemingly mundane materials like steel, concrete and plastic, to sophisticated materials like silicon, carbon nanotubes and light-emitting polymers. But what science underlies the making and the design of these materials? While physics and chemistry are important, Materials Science looks into a different and unique place — the microstructure –of a material. In this course, you will learn how the properties of a material are influenced by its underlying structure, and how we can control this structure using processing. By the end of the course, you will see your smart phone, your car, your home, and your world, through different eyes.
Today, over 160,000 materials exist, and we interact with a wide range of them every day without giving a second thought to how they work. Materials Science provides us with a framework to understand how materials work and how we can control the various properties that make them useful to us. It is a mash-up of physics, chemistry, and mathematics that produces the materials that enable every physical structure we use.
Pre-Requisite: None
Professor: Varies
This course opened my eyes to a very interesting field and taught me many concepts that will be very useful in my later years here at UVA. Even though this course was difficult because of the complex topics the class managed to keep my interest the entire time and with all the available help it allowed me to learn many concepts that I originally did not understand at all.
MSE 2090 has been the most interesting course I have taken in the past few years. I truly appreciate the opportunity to have passionate professors who care about what they teach as well as our understanding of the concepts. For instance, all of the instructors hold office hours throughout the week, making it easy to get additional support outside of class. Although I personally find the content challenging, I have really enjoyed learning about completely novel topics in a very detailed manner.
Materials are valuable to us when they serve a useful purpose - they carry, they contain, they conduct, they transmit, they resist, they compute, they adorn, they save, and yes, they kill. How well a material performs in YOUR application depends on its relevant properties. Materials properties are a bit like human behaviors: they vary greatly from material to material, and in an individual material, they can be modified and improved, albeit with effort. How do we do that? And how do we accurately measure what we've done? That's what MSI: Properties is all about. This is your opportunity to measure key properties in common and important materials. You will also get to change the internal structure of your materials, to see firsthand how the properties depend on structure. This will deepen your appreciation of the process-structure-properties design paradigm of Materials Science. MSI:P will help you become: a better experimentalist and observationalist, a thoughtful interpreter of data, and a more confident communicator of your accomplishments.
Pre- or Co-Requisite: MSE 2090 Introduction to MSE
MSE 2101 not only offered me an opportunity to familiarize myself with tools and techniques used for materials properties but also fostered an environment where I grew my scientific writing and presentation skills. It is a class that deeply cultivates students' growth as professionals and future materials leaders. Anisha Jarang, MSE Major

I liked getting to know my MSE classmates in 2101 progressively during each lab. Being a small major, you get to learn about everyone's interests and passions while working in the lab and gain new perspectives on an MSE career from the TAs and professors. Adrian Motas, MSE Major


Why does a dropped ceramic mug shatter, but a metal one does not? Where do gemstones get their colors? Out of the many plastics out there, why do some soften when becoming hot? How do magnets really work? You will find answer to questions like these in MSE 2090, where we discuss how combinations and arrangement of atoms give rise to myriads of mechanical, electrical, optical, and magnetic properties in various materials, and how they are utilized in engineering, science, and design.
In this course we start from a brief review of classical thermodynamics, which is not only necessary for understanding of the phase transformations in materials but, arguably, may provide the philosophical basis for understanding of Natural Laws. To get a solid physical/intuitive understanding of basic thermodynamic concepts, we will undertake several forays into statistical thermodynamics, and discuss the meaning of the heat capacity, internal energy, enthalpy, entropy, and free energy. We will then apply the thermodynamic concepts to the analysis of phase equilibria and phase transformations in one-component and multi-component systems. We will learn how to read and analyze phase diagrams of real materials and how to construct phase diagrams from thermodynamic data. Examples illustrating the connections between the phase diagrams and microstructure/properties of real materials will be discussed. Finally, we will discuss the thermodynamics of interfaces between solid, liquid, and vapor phases, and consider the role the interfaces play in phase transformations and in defining the equilibrium shapes of crystals and elements of material microstructure.
Enrollment Requirements: Students must have completed APMA 2120 or MATH 2310 or MATH 2315.
This course is challenging but teaches you the fundamental thermodynamics in materials in rigorous details.
I thoroughly enjoyed this course. I had to work hard to take notes and complete the homework, but the homework truly prepared me for the exams. At times it was challenging and the material was difficult but overall, I enjoyed it and it increased my interest in material science.
.gif)
The properties of important and useful materials derive from their structure, and structure is what MSE 3060 is all about. We begin with how the atoms themselves arrange – most often, in the form of crystals. Crystal structures can be very simple, or sublimely complex. We cover some basic approaches that let us rationalize, understand, visualize, and even calculate upon, all crystal structures. Next we look at how diffraction – of x-rays, electrons or neutrons – allows us to determine the crystal structure of any material.
While crystal structure is elegant and even beautiful in its conception of perfection, it is the defects in the crystalline structure of engineering materials that lends ‘personality’ to each material. Defects lead to variability in the properties of a material, and variability provides an opportunity to engineer and refine the properties to meet the needs of our applications and technologies. We examine different classes of defects, including surfaces and interfaces, dislocations, and point defects. Broadly, the collection of defects in a material constitutes its microstructure.
MSE 3060 connects to most other materials science courses. In connects to Thermodynamics in that every solid phase is in part defined by its crystal structure. It connects to Kinetics through the atomic-scale movements via defects that underlie diffusion, and in terms of phase transformations. In connects to properties courses (Mechanical, EMOP) since crystal structure and microstructure beget properties. And it connects to Processing, since we use processing to obtain the microstructures we need.
Enrollment Requirements: Students must have completed APMA 2120 or MATH 2310 or MATH 2315.
Thermodynamics tells you why a process should happen, and kinetics tells you how. In this course, you will gain a deeper understanding about the mechanism and rate of phase transformation, one of the most beautiful phenomena in the world of MSE. This course is a great synthesis of MSE core subjects: crystal structure, thermodynamics, and defects.
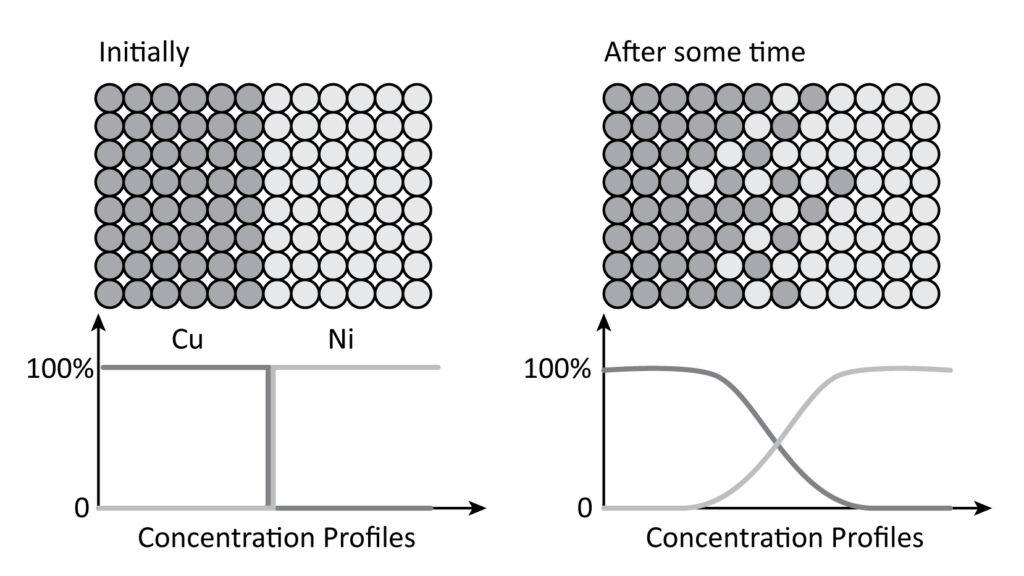
This course will cover three main topics: 1) chemical reaction kinetics, 2) diffusion (mass transport), 3) phase transformation and microstructure evolution. We will build on the understanding of thermodynamic driving forces and of phase diagrams you developed in MSE 3050 and apply these concepts to the analysis of the key kinetic processes, phase transformations, and the development of microstructure in materials. We will start by discussing chemical reaction kinetics in the gas phase. Most kinetic phenomena in condensed matter involve diffusion, and we will consider the phenomenological descriptions as well as atomic-scale mechanisms of diffusion in materials. We will then discuss the kinetics of phase transformations and microstructure evolution, including the classical nucleation theory, mechanisms of growth and coarsening, theory of capillary, grain growth and so on. By the end of the course, we will see how the interplay of thermodynamic driving forces and kinetics of mass transfer defines the formation of complex microstructures of real materials.
Pre-Requisite: MSE 3050 Thermodynamics and Phase Equilibria of Materials
My favorite part of learning is when the material I learn becomes second–nature, when I encounter problems in the real world pertaining to the topic, my brain is able to naturally relate it to my prior knowledge. This course accomplished that feeling, and for that I'm grateful.
I think this class was structured the best out of any class I've had at UVA and that's not an exaggeration.
Prof Zhou was always open to questions and never shut down any perspectives.

In MSE 2090 you learned about materials phases, crystal structures, and microstructures. You might have asked the following questions after that course: How do we know the atoms sit in those locations? How do we know that this material is this phase? How do we know that grains exist and what grain sizes are? In MSE 3101 we answer those questions and more. This hands-on laboratory course will teach you how to observe different phases and grains using a light microscope. It will teach you how to use an electron microscope to identify regions with different phases and even smaller microstructure features than you can see with light. You will learn how an electron microscope can be used to tell you the elements that comprise a material. Finally, you will learn how X-rays can be used to identify phases, where the atoms sit in a crystal, and even tell you what fractions of a material are different phases. Armed with this knowledge, this course will allow you to use all these tools to investigate an object and determine what materials are within, how it was made, and why those materials were selected for the given application.
Pre-Requisite: MSE 2090 Introduction to MSE
What is this course?
• Why were there no cell phones in 1970?
• What device will our next generation not be able to survive without, but that hasn’t yet been invented?
• How are these devices possible?
Devices based on the electrical, magnetic, and optical materials are a huge part of our lives. If you accessed this syllabus from the internet, then you used an electronic device (computer, cell phone) that interfaces with optoelectronics (the screen) to allow you to view this document. If the device has a disk drive then the motor functions due to the electromagnetic properties of the materials.
The overall goal of this course is to understand why materials have electronic, magnetic, or optical properties, and how these can be manipulated. In materials science we call this the process-structure-property relationship. We will investigate the underlying physics behind these properties and also how they lead to exciting applications. With the fundamental properties understood, we will explore the material combinations and interfaces that make modern nanoelectronics and optoelectronics possible.
Pre-Requisite: PHYS 2415 or equivalent

Nanoscale Science and Technology is an introduction to the rapidly evolving world of nanoscience. It uses the materials perspective to build a comprehensive basis which we use to understand the many different aspects of nanoscience. We begin with the seemingly simple question “what changes if we move from the macroscale to the nanoscale?” and consider electronic, optical and mechanical properties. At the nanoscale an additional component is dimensionality which leads us to compare nanowires (one dimensional), quantum dots as artificial atoms, and 2D materials. The 2D materials have only emerged in the last decade as powerful materials to build new electronic devices, catalysts, and composites. The course focusses on materials with nanoscale dimensions which are of particular interest for harnessing their properties. We study the synthesis of nanomaterials, their properties and application and will learn along the way quantum mechanics, structure, thermodynamics, growth methods, device fabrication and characterization methods which are needed to work at the nanoscale. This course integrates the current research literature, and numerous up to date examples. Tasks will include research papers, critical reading, simulations, traditional HW problems to practice the underlying physical and chemical concepts, peer review, and presentations. On occasion we will invite experts in a specific field of research like synthesis of quantum dots, or 2D devices.
Pre-Requisities: Must have completed MSE 3670 (EMOP)

This course examines the fundamental principles of physics, chemistry, materials science, and manufacturing which underlie the making, shaping, and fabrication of engineering components from casting and deformation processing of metals, to powder processing of metals and ceramics, to polymer injection molding, to thin-film processing and lithography relevant to microelectronic circuit fabrication.
Pre-Requisites: MSE 3070
Why take the course on atomistic modeling as an undergraduate?
• Combine your interest in Computer Science, Physics, Chemistry, Mathematics, with the fascinating world of Materials Science and Engineering
• Learn how to translate theory/equations to clever algorithms and efficient codes.
• Take advantage of the UVA Advanced Research Computing Services – from visualization to high-performance computing.
The course introduces students to atomic-level computational methods commonly used in Materials Science, Physics, Chemistry, and Mechanical Engineering. The molecular dynamics and Monte Carlo methods are discussed in depth, from the introduction to the basic concepts to the overview of the current state-of-the-art. The emphasis of the course is on getting practical experience in designing and performing computer simulations. Students use and modify the pre-written codes and write their own simulation and data analysis codes while working on their homework assignments and term projects. The results of term projects are presented at a mini-symposium held at the end of the class.
Course web site: https://CompMat.org/mse6270/
Enrollment Requirements: must be 3rd or 4th year Engineering student
The simulation is very important perspective in terms of research today. I found this class is very challenging and interesting. I learned a lot from the homework by tinkering around the code and finding reasons why and how the phenomena happened.
The projects are a very cool way to hammer down fundamental thermodynamic and physics concepts.
I recommend every student who wants to do simulation work should take this class!

The overarching objective of this course is to develop understanding of the phenomenology and fundamental principles describing the origins of material deformation and fracture in response to mechanical loading. Breadth of mechanical behavior and structural materials are emphasized over depth, which is covered in upper division courses. Engineering and scientific principles are integrated in an approach that includes: (a) governing continuum mechanics equations, (b) materials science principles at the atomic defect to microstructure scale, (c) examples of property phenomenology, (d) descriptions of test methods, and (e) problem solving to reinforce concepts and demonstrate applications. Each important mode of mechanical behavior is discussed in the context of a specific material; structure-property relationships are identified. After an introduction to elasticity, plastic deformation is considered based on dislocation concepts, leading to an understanding of strengthening mechanisms. Fracture is examined based on continuum fracture mechanics and microstructure damage leading to toughening mechanisms. Cycle-dependent (fatigue) and time-dependent (creep) plastic deformation processes are described and subcritical crack growth is discussed as the result of deformation-environment interactions. Modern predictive approaches are highlighted and the foundation for property modeling and component simulation is established.
Enrollment Requirements: Must have completed MSE 3060.
The capstone experience consists of two components. One component, which relates to the project that the students will perform (MSE 4592 Capstone 1 and 2) will be guided and technically evaluated by faculty from the Material Science and Engineering (MSE) department. The other component covers the science, technology, and society aspects of the students’ chosen project, as well as aspects of style, writing and ethics will be taught by the Department of Engineering and Society. The MSE faculty will work collaboratively with each other and the students to develop a range of projects to demonstrate students’ knowledge and skill in materials science and engineering and communication as the students apply their skills to real-world engineering problems at home or abroad. The design projects may range from company-sponsored projects to contextual engineering challenges that are facing society. Working with the faculty members, students will work in teams to produce a thesis prospectus in the fall, followed by a complete technical thesis in the spring. The capstone experience will culminate with a final report and formal presentation.
